The COVID-19 Vaccine
November 30, 2020
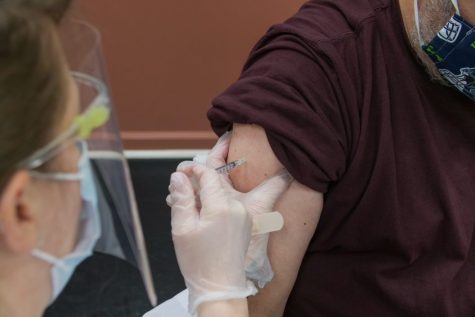
With coronavirus cases in the US constantly rising, the race is on to find an effective vaccine to fight the virus. There is no authorized or recommended vaccine yet, as all of the vaccines in the making are still in Phase 3, which is full of clinical trials and testing. There are five vaccines currently in Phase 3, and the Center for Disease Control is expecting the first mRNA COVID-19 vaccines to be authorized for use in the US in the next month.
mRNA vaccines are new but not unheard of, and researchers have been studying them for decades. While there are no mRNA vaccines authorized for use in the US yet, the COVID-19 vaccine will likely be the first. Unlike many vaccines, which put a weakened or dead copy of the infectious disease in the body, the mRNA vaccine teaches the body to make a protein that triggers an immune response, which is what produces antibodies and protects us from getting infected. A perk of the mRNA vaccine is that it can be made faster than the traditional method of making vaccines, which will be incredibly helpful in fighting the ever-spreading pandemic.
Some concerns are whether the vaccine will give the patient the virus and whether it will interact with or affect our DNA in any way. The answer to both of these questions is no. The vaccine does not use the live virus that causes COVID-19, and the mRNA involved in the vaccines never enters the nucleus of the cells, which break down the mRNA as soon as it is finished learning how to protect the body from infection. The COVID-19 vaccine will be held to the same standards as every other vaccine authorized for use in the USA, and the only vaccines that will be authorized are the ones that meet every single safety criteria the Food and Drug Administration has.
The issue with the coronavirus is that it is constantly evolving and will continue to do so after the vaccine is authorized for use. Another virus like this is the flu, which is why the Center for Disease Control recommends getting a flu shot every year. The flu shot is updated yearly to protect against the new evolved strands, and it is quite likely the COVID-19 vaccine will be similar, as the virus continuously evolves. The COVID-19 virus we have now is most definitely not the same as the one that first reared its ugly face last winter.
The COVID-19 vaccine is something the US desperately needs, and we are seeing some hopeful possibilities with the five vaccines in the clinical trial stage, one of which was funded by Dolly Parton herself! Mrs. Parton donated $1m to Moderna and encouraged her fans to donate as well. Her fans raised over $100,000 for the vaccine, and Dr. Anthony Fauci, a top disease specialist in the United States, said the promising vaccine could be available as early as late December 2020. For more information on the COVID vaccine and Mrs. Parton’s help with it, go to https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/mRNA.html or https://www.cnn.com/2020/11/17/entertainment/dolly-parton-covid-moderna-vaccine-trnd/index.html
Kaitlyn Willett is a Dakota Student News Writer. She can be reached at [email protected]